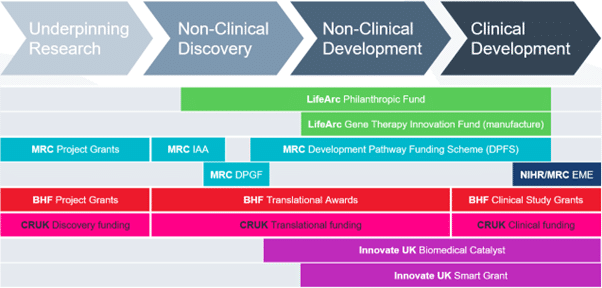
Funding: LifeArc gene therapy innovation fund
The LifeArc Gene Therapy Innovation Fund is a £5m annual fund to support manufacture of viral vector at the world-class network of Innovation Hubs for Gene Therapies as part of LifeArc’s Rare Disease Translational Challenge.

Overview
The Gene Therapy Innovation Fund (GTIF) aims to support the advance of promising gene therapies for people living with rare conditions. There will be no expectation of a return to LifeArc on this funding, but projects must have a credible technical and financial path to significant patient benefit if they are successful. The GTIF is a £5M annual fund. The funding panel reviews full applications and makes funding decisions twice a year.
The fund provides grants to academic researchers working to advance new gene therapies and who require viral vector material manufactured to GMP (or GMP-like) grade in order to support the programme. Funding is awarded for academics to use the manufacturing capabilities of the Innovation Hubs for Gene Therapies, and closely associated work. This is likely to include manufacturing process development, process scale-up, development of analytical methods, production of technical batches and batches manufactured in accordance with Good Manufacturing Practice (“GMP”) guidelines, to support regulatory studies and/or clinical trials. Where projects include substantial non-clinical and/or clinical studies, it is expected that applicants will secure co-funding from a suitable scheme, such as LifeArc’s Philanthropic Fund, to cover the wider translational aspects of their project, e.g., GLP safety/toxicity and/or clinical trial costs. Where the manufacturing aspects constitute part of a larger project, funding under GTIF may be conditional upon securing suitable co-funding.
Funding available
Funding is currently open
If you would like to find out more about the Gene Therapy Innovation Fund or are considering submitting a preliminary application, we ask that you contact the funding managers Joanna Davidge and Nick Darvill. Funding managers will be in contact for further discussion if your proposed project fits within the scope of the GTIF.
Application eligibility
- Applications should be for an academic-led gene therapy project requiring manufacturing services that can be provided by one of the Innovation Hubs for Gene Therapies.
- Applications may involve a collaboration with other academic partners or industry. Any non-academic partners must provide identifiable project support and appropriate collaboration agreements or heads of terms should be in place.
- The project must fit within the scope of LifeArc’s Rare Disease Translational Challenge, and so the must address a significant, unmet healthcare need for a rare disease or condition.
- The project must have a strong scientific rationale, be target-driven, with a credible delivery plan and milestones, and have a clear route to patients. It must be at an appropriate stage of development to benefit from the expertise of the Innovation Hubs, i.e., be suitably advanced to justify expert manufacture services.
- We encourage applications that include co-funding from other LifeArc funds, other funders, or medical research charities.
- The funding is philanthropic and LifeArc does not ask for any return on revenue, or a share of intellectual property. The academic lead must demonstrate access to required background IP and freedom to operate for the therapy to be developed.
- Lead applicants may only submit one application per round.
Application and funding process
Pre-application
You are welcome to contact us before submitting your preliminary application to ensure your project is in scope and discuss any matters such as co-funding. Please contact the funding managers Nick Darvill or Joanna Davidge.
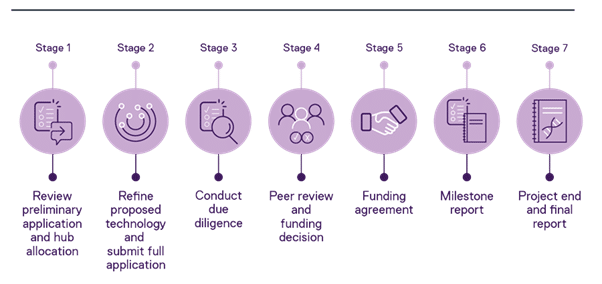
Stage 1: Preliminary application and Hub allocation
Preliminary applications can be submitted at any time and are reviewed 2-3 times per year. LifeArc will review preliminary applications to ensure they are eligible for the Fund. The project will be matched to an Innovation Hub with appropriate capabilities and capacity by the Allocation Panel.
Stage 2: Develop and submit full application
LifeArc and the allocated Innovation Hub will work with the applicants to address any identified gaps before submitting a full application. In a process that will be tailored to the needs of the respective application, we will assist you to expand and refine the proposal in preparation for your submission. Full applications are reviewed twice per year, with March and September deadlines.
Stage 3: Due diligence
Once the full proposal is submitted, LifeArc will conduct due diligence on the information provided and gain external peer review. Comments will be shared with applicants who will be able to submit responses before the proposal is assessed at panel.
Stage 4: Peer review and funding decision
The funding panel will consider the submitted application, diligence, peer review, and applicant responses and make the final decision. The panel makes these project decisions twice a year, usually in June and December.
Stage 5: Funding agreement
If the panel agrees to fund the project, LifeArc will formalise the funding agreements and project milestones. If a proposal is declined, feedback will be provided for every application.
Stage 6: Milestone reports
On award, a reporting schedule will be agreed with the awardee. LifeArc and the Hub will monitor progress and will be available for support and advice where appropriate.
Stage 7: Project end and final report
The results of the project are reviewed, and the next steps discussed with the network of Innovation Hubs for Gene Therapy and LifeArc.
Allocation panel
The allocation committee is formed of scientific and business development representatives from across the three Hubs, along with a LifeArc representative. The allocation committee will recommend the most appropriate Hub to support the project matching the project’s manufacturing needs to their individual capabilities. You may indicate a preferred Hub if you wish, but one will be assigned to you if you do not specify a preference in the preliminary application.
Funding review panel
All applications will be reviewed by our funding panel, consisting of external expert partners from academia, charities, and industry. Read about our funding panel members here.
FAQs
When is the closing date?
The next deadlines for preliminary applications are:
- 8 May 2025
- 13 November 2025
How do I submit a preliminary application?
If you would like to submit a preliminary application, please contact the funding managers Joanna Davidge and Nick Darvill or get in touch using the form below. Funding managers will be in contact for further discussion if your proposed project fits within the scope of the GTIF. Funding managers may then provide access to the preliminary application form for you to complete.
When will I hear the outcome of my preliminary application?
You should hear the result of your preliminary application within one month of the preceding deadline, often sooner.
How do I submit my full application?
Submission of a full application to the LifeArc Gene Therapy Innovation Fund is by invitation only, and with the support of the allocated Innovation Hub.
When is my invited full application due?
Full applications can be submitted at any time after allocation to a Hub. The funding panel reviews full applications and makes funding decisions twice a year, in June and December. The closing date for completed, full applications to be considered at the next panel will be in March and September each year.
Do I have to pay the Hubs for the work?
The initial proposal development with LifeArc and the allocated Hub is provided free of charge. Using their expertise, the allocated Hub will assist the academic applicant to deliver on a development plan with a view to the proposed project being suitably ready to the submission of a full application.
How do you assess projects for funding?
Funding decisions are based on consideration of unmet medical need, strength of the scientific rationale and supporting data and there being a clear and feasible path to patient benefit, and suitability for charitable funding. Financial, ethical, and scientific due diligence and peer review are carried out to ensure the review panel is satisfied as to the legal and technical acceptability of the research, researchers and organisation involved in carrying out the project.
What are the reporting requirements?
Awardees are required to provide a quarterly update report from the project commencement date, alongside agreed milestone dates.
Can I submit a proposal that requires co-funding?
Co-funded applications are welcome with the appropriate collaboration terms outlined. Funding support will be awarded to the academic institution.
Does LifeArc expect a revenue share?
LifeArc will not ask for any financial return or intellectual property from projects supported by this grant, allowing researchers to focus purely on delivering a result that benefits patients.
What happens if I am unsuccessful the first time? Can I apply again?
We will provide feedback with the funding decision. Any invitation to reapply will be made at the funding panel’s discretion.
Is funding available to companies or to academics working with a company?
The funding is available to develop assets and IP for which the rights belong to academic/non-profit organisations, such as a university. Proposals to support the development of therapeutics for which the rights belong to a company (such as a spinout), including via licensing or assignment from a university, will not be eligible.
If you are unsure, please contact us with a description of your circumstances and we can advise on your specific situation.
Contact us
In submitting your personal data via this form, you consent to being contacted via the details provided so that your enquiry can be responded to. If you would like your data to be removed, please email dataprivacy@lifearc.org.
Please see our Privacy Policy in relation to the personal data you submit to us through this page.
Latest news
-
Third global Longitude Prize to be launched in 2025 focusing on ALS
-

LifeArc appoints Chief Medical Officer to deliver more scientific breakthroughs for patients
-

£2.7m infectious disease fund to boost rapid testing, drug trials and innovative specimen storage
