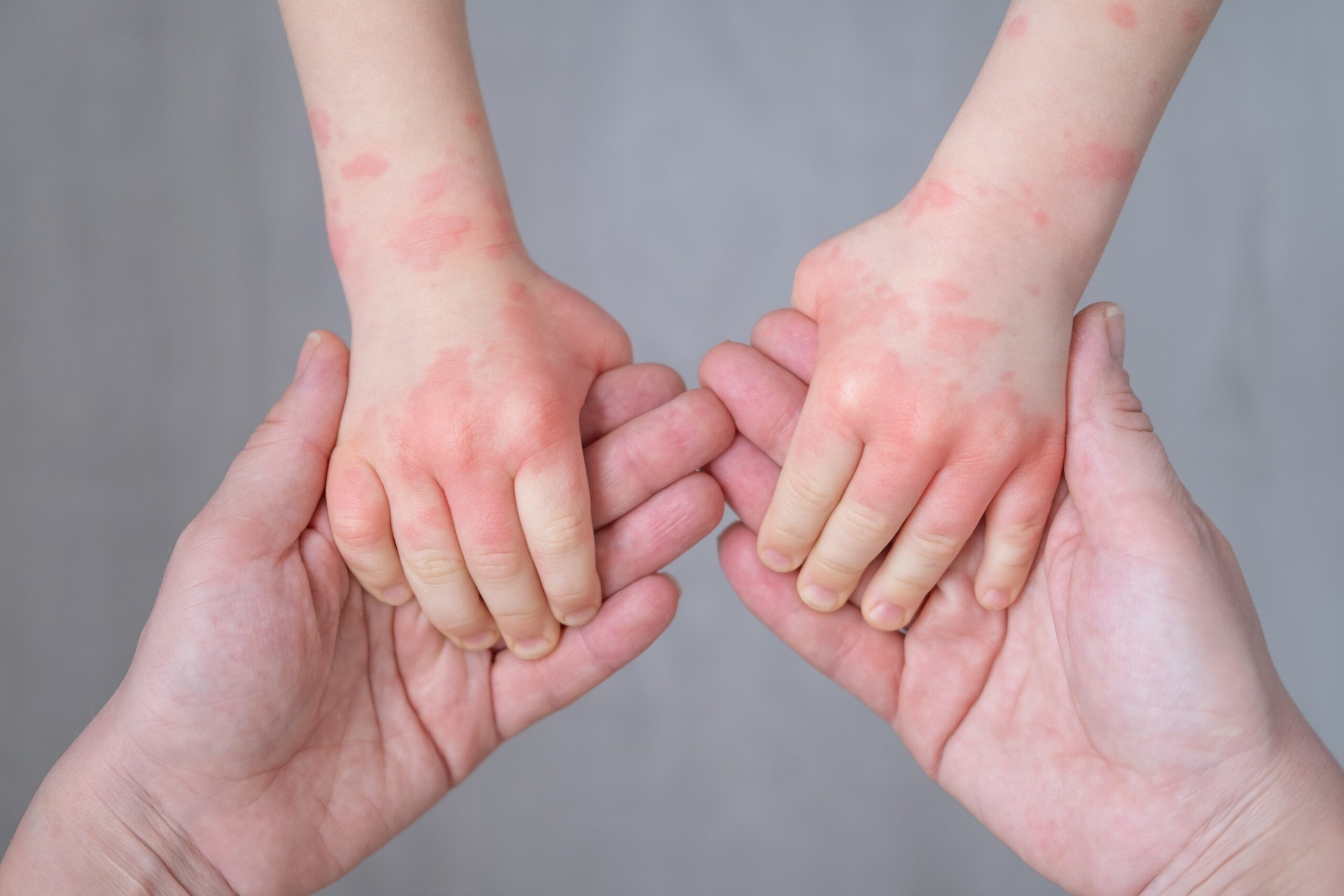
An antibody we humanized has entered early-phase clinical trials for the most common type of eczema – facilitated by collaboration across the translational cascade.

Overview
In collaboration with Dr Andrew McKenzie, MRC Laboratory of Molecular Biology, we humanized the world’s first anti-IL17BR antibody – which has the potential to treat a wide range of inflammatory conditions.
Following humanization, we facilitated licensing of the antibody to Hong Kong-based pharmaceutical company SinoMab BioScience Limited, who led the antibody’s downstream development and worked closely with the academic team to understand the extent of its applications.
SinoMab is now testing the antibody in a Phase 1b trial in people with atopic dermatitis, the most common form of eczema.

At SinoMab, we have a passion for science and a mission to innovate, not just follow the crowd.
LifeArc’s humanized anti-IL17BR antibody immediately got our attention. As a scientist, I was fascinated by the rationale behind it, and we realised it was an overlooked gem with huge potential – worth taking on the developmental risk for. I truly believe that if this antibody reaches the clinic, it could be one of the world’s top drugs.
Another thing that drew us to working with LifeArc was the team’s vision. It’s not just about making a profit – it’s about doing good for humanity and translating science in practical ways to help patients.
Dr Shawn Leung, founder, chairman and chief executive officer, SinoMab BioScience Limited
The unmet need in atopic dermatitis
At least 204 million people – 2.6% of the world’s population – are affected by atopic dermatitis, the most common form of eczema. While treatments exist, including corticosteroids and the antibody dupilumab, not all patients respond, and they either can cause significant side effects or are slow in their anti-itch effect.
There’s huge potential for a new drug that meets 3 important criteria: safety and tolerability, fast-acting anti-itch effects, and effective skin healing.
In 2024, the antibody that we humanized, SM17, entered phase 1b clinical trials as a first-in-class treatment for atopic dermatitis.
Having overcome multiple hurdles, SM17’s position within the drug development pipeline is a testament to a unique model of collaboration across the translational cascade, facilitated by our position within the research ecosystem. This enabled close collaboration with both the academic team and pharmaceutical company for humanization and onward development, respectively, to bring SM17 closer to patients.
Targeting the inflammatory response
Dr Andrew McKenzie, of the MRC Laboratory of Molecular Biology in Cambridge, discovered a type of immune cell that contributes to tissue homeostasis, called group 2 innate lymphoid cells (ILC2s).
Normally, these cells detect tissue damage and infection, regulating the immune response by releasing inflammatory cytokines and activating the immune system. In many chronic conditions, however, ILC2s are overactive or found in higher levels, causing inflammation.
Blocking a receptor on the surface of ILC2s, called interleukin-17 receptor B (IL17BR), blocks the production of key cytokines and can achieve a rapid, anti-inflammatory response.
We collaborated with Andrew’s team to generate a clinical candidate antibody against IL17BR. The project was split into two phases: generating a mouse monoclonal antibody, followed by humanization of the lead candidate.
“Humanizing the antibody wasn’t straightforward,” shares Dr Seema Patel, principal scientist at LifeArc and the lead scientist for the humanization phase. This could have been the end of the road – but, driven by our belief in the antibody’s potential, the team persevered.
“We had to perform additional engineering to improve the expression levels and developability profile of the antibody,” she explains.
The result was a humanized antibody against IL17BR that matched the expression of the parent molecule and had promising characteristics for onward development. The humanized antibody officially received its patent in Europe in 2024.
Properties of the antibody:
- Parent species: mouse
- Target: IL17BR
- Outcome: expression, affinity and stability to match parent antibody
Finding the right partner for onward development
We originally developed the antibody with other inflammatory conditions in mind, with a particular focus on asthma. But following humanization, we hit another hurdle: despite promising preclinical research, companies were hesitant to take on the unknowns of interfering with the downstream effects of cytokines.
Enter SinoMab BioScience Limited, a biopharmaceutical company based in Hong Kong and headed up by Dr Shawn Leung.
“We’re constantly on the lookout for new targets with great potential and the IL17RB project immediately got our attention,” he expands. “In addition to the science behind the IL17RB target, I really liked LifeArc’s vision. It wasn’t about making a profit – but bringing science into practical use to help patients.”
In 2019, SinoMab came on board to license that antibody for further development, naming it SM17 after SinoMab switched the isotype to human IgG4.
Following extensive research into the humanized antibody by SinoMab, and into the mouse antibody by Andrew’s team in Cambridge, a full list of potential indications was uncovered – including asthma, fibrosis and, eventually, atopic dermatitis.
“Working in this way and learning from each other, facilitated by LifeArc, has produced a positive feedback loop,” says Shawn. “Together, we’ve learned much more about SM17 and found new directions to pursue. It’s been very effective.”
A new treatment for atopic dermatitis?
“There were – and still are – no other companies working on antibodies that target IL17RB. This brings some risk, especially for a small company like ours,” describes Shawn. “But at SinoMab we have a mission to innovate, not just follow the crowd.
SM17 was one of the first antibodies to go through LifeArc’s comprehensive suite of biophysical assays, providing an assessment of affinity and developability, as well as insight into safety and tolerability. LifeArc continues to use these assays in our antibody discovery and humanization platforms, giving antibodies the best chance of progressing to the clinic.
Fuelled by these assays and extensive preclinical research, SinoMab tested SM17 in a first-in-human trial with healthy individuals in 2022. The drug was well-tolerated, with no severe adverse effects.
SinoMab began testing SM17 in a Phase 1b trial for atopic dermatitis in 2024, with the first patient dosed in China in June. Initial observations look promising – and Shawn is optimistic that SM17 could meet the 3 key characteristics of safety, rapid anti-itching and effective skin healing.
“SM17 has huge prospects,” he says. “We believe that, with its first-in-class advantage, SM17 will provide a new and effective treatment option for atopic dermatitis, benefiting more patients.”
Partner with us to humanize your antibody
We have a breadth of experience in antibody humanization and consider any target, therapeutic indication or modality.
Contact us
In submitting your personal data via this form, you consent to being contacted via the details provided so that your enquiry can be responded to. If you would like your data to be removed, please email info@lifearc.org.
Please see our Privacy Policy in relation to the personal data you submit to us through this page.